On July 8, 2022, Sinotau’s new drug application for florbetaben F18 injection (NeuraceqTM) was accepted by CDE.
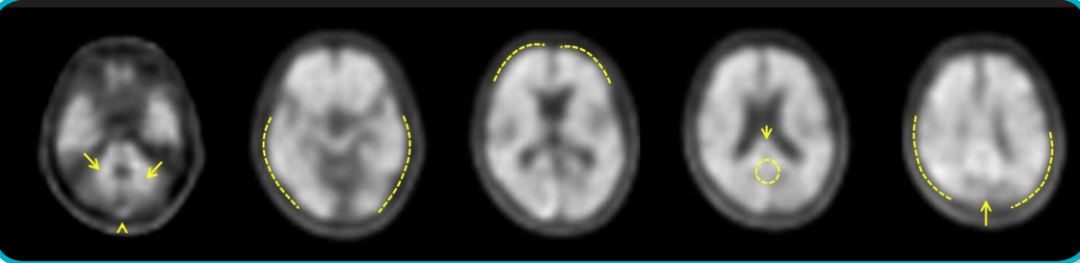
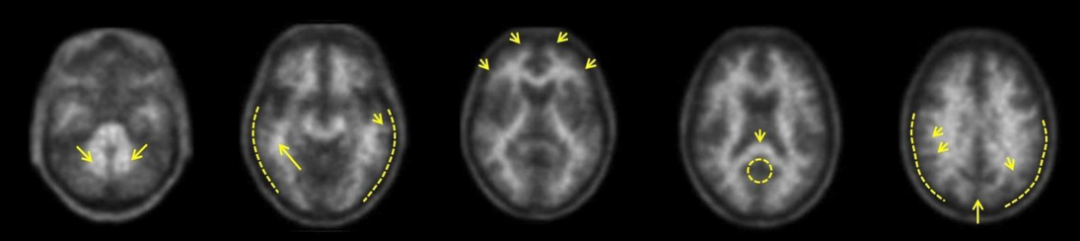
Neuraceq is a brain β-amyloid (Aβ) positron emission tomography (PET) imaging agent developed by Life Molecular Imaging (LMI) in Germany. It allows for the accurate measurement of β-amyloid (Aβ) levels in the brain of adult patients through PET/CT or PET/MRI to assess the cause of cognitive decline in Alzheimer's disease (AD).
The products were widely used in AD diagnosis after first being approved for marketing in Europe and the U.S. Sinotau obtained exclusive permission for R&D, registration application and commercial production in China, and then actively promoted domestic clinical trials and registration, and was accepted for drug application by CDE on July 8, 2022.
Florbetaben F18 is an 18F-labeled stilbene derivative that binds specifically to Aβ plaques in the cerebral cortex of AD patients and does not interact with Tau or α-synuclein in tissues. Florbetaben F18 generates positron signals through 18F, and PET scans are performed by PET/CT or PET/MRI to detect Aβ plaque formation signals in various brain regions with PET images.
Aβ plaques may be deposited in the brain as early as 15-20 years before the appearance of dementia symptoms, and with the prolongation of the disease course of cognitive impairment, the deposition of Aβ plaques in the brain gradually increases. At present, worldwide, especially the U.S. FDA approved clinical stage or has been approved AD therapeutic drugs, are AD early stage to ensure the effectiveness of treatment, slow down or even reverse the disease process.
The acceptance of the marketing application for florbetaben F18 heralds a new option and hope for AD patients in China, representing the first platform product in the field of precise diagnosis and concomitant diagnosis of AD in the future, which is a historical breakthrough for the integration of AD disease diagnosis and treatment.
Currently, more than 55 million people suffer from Alzheimer's disease worldwide, with more than 10 million of them located in China. The awareness and attention of Alzheimer's disease among Chinese residents are low, and low diagnosis rates (especially early diagnosis) and low treatment rates are common. It takes an average of 8-10 years for Alzheimer's disease patients to progress from mild to severe, while it only takes an average of 2-4 years to progress from mild cognitive impairment to mild dementia. Effective grasp of this golden window for treatment, timely diagnosis and therapeutic intervention can greatly slow down the rate of disease progression.
National policies are also constantly clarifying the goal of reducing the prevalence rate. The "Special Service Work Program for Exploring Prevention and Treatment of Alzheimer's Disease" issued by the National Health Care Commission emphasizes that the public awareness rate of Alzheimer's disease prevention and treatment in China should reach 80% , and the cognitive function screening rate of elderly people in the community (village) should reach 80%. The future approval of the product for marketing has special significance for clinicians that will make a better diagnose for AD disease, assess the risk of disease in patients' families, or in areas such as high-end medical checkups. It is the only commercial application for quite some time in the future and is well worth looking forward to.