On September 28, Beijing time, Biogen and Eisai, foreign companies dedicated to the R&D of Alzheimer's disease (AD) drugs, announced that its new AD drug Lecanemab Phase III clinical trial met its primary endpoint, which means that the drug can effectively improve patients' cognitive ability and slow down the progression of AD. Lecanemab is the first beta-amyloid (Aβ) antibody to achieve positive results in a confirmatory Phase III trial, which is a milestone. The news quickly ignited the industry and gave hope to AD-related researchers, AD patients and families.
The theme of this year's World Alzheimer's Day, “Know Dementia, Know Alzheimer’s” is even more meaningful with the breakthrough in AD treatment drugs.
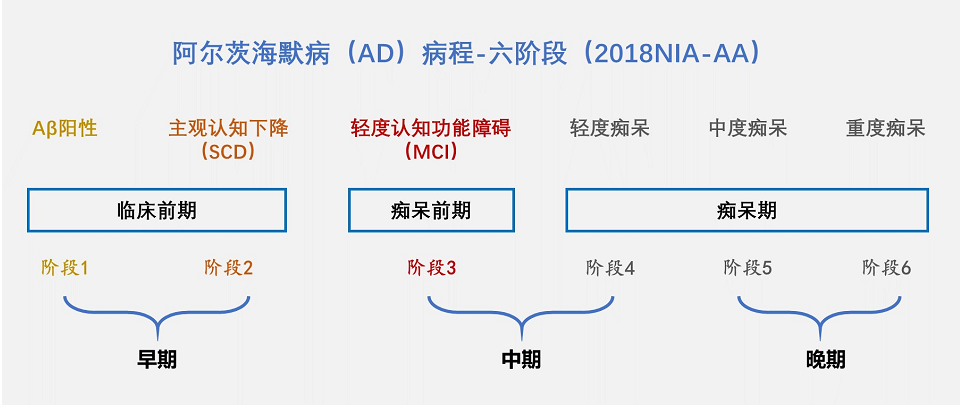
The course of AD consists of six stages (figure below). Stages 1 and 2 have no obvious symptoms of cognitive decline and are difficult to detect. It is of concern that Aβ deposits have already appeared in the brain by this time. And by the time the disease progresses to the appearance of obvious symptoms (stages 3, 4, 5 and 6), Aβ deposits in the brain may have been present for up to 20 years.
The earlier the AD therapeutic drugs are applied, the better the effect. So it is more important to "early prevention and early treatment". The key is early detection and early diagnosis, in order to reverse the disease before brain cell necrosis, so that patients don’t develop the disease for life.
Current methods for early diagnosis of AD include Aβ testing by obtaining cerebrospinal fluid through lumbar puncture and Aβ testing by positron emission computed tomography (PET). Lumbar puncture iore difficult for paties an invasive test, which is mnts and families to accept, while PET is not only non-invasive, but also the most accurate.
Aβ detection by PET requires intravenous injection of Aβ imaging agent, but there is no Aβ imaging agent that can be widely used in clinical application in China. In order to fill this clinical gap and meet the needs of AD patients, Sinotau has conducted the R&D of Aβ-PET imaging agent - florbetaben F18, and has submitted a new drug application (NDA), which is the only Aβ-PET imaging agent that has submitted an NDA. It is expected that florbetaben F18 will be widely used in the clinic next year.
Florbetaben F18 is an Aβ-PET imaging agent used in brain scans of patients with cognitive memory problems, so that doctors can see if they have large amounts of beta-amyloid plaques in their brains. This product has been marketed in Europe and America, and will be the first Aβ-PET imaging agent that can be applied to AD diagnosis in China. It can be used not only for the early diagnosis of AD, but also has been widely used abroad for patients screening and efficacy assessment in clinical trials of AD therapeutics.
The future clinical application of these AD therapeutics will not be possible without the accurate diagnosis of Aβ-PET imaging agents. The close collaboration between precision diagnosis and effective therapy will provide a comprehensive solution to the challenge of early diagnosis and reversal of AD.
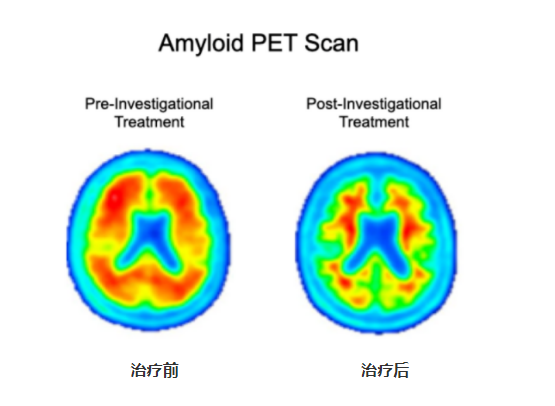
Amyloid PET imaging scans from a representative participant in the Phase II clinical trial of Lecanemab
The image on the left is taken before the participant has started on the investigational treatment. The image on the right is taken after 18 months of investigational treatment with Lecanemab. Aβ-PET imaging can accurately, qualitatively and quantitatively measure the level of amyloid plaques in the brain. PET qualitative readings showed a significant reduction of amyloid plaque burden in the brain compared to the pre-treatment period, and PET quantitative analysis showed 0.306(22%) reduction in SUVR after treatment.
(image resource: https://www.aheadstudy.org/participation-requirements/)
About Sinotau Pharmaceutical Group
Sinotau Pharmaceutical Group officially launched the R&D of new generation radiopharmaceuticals in 2014. Sinotau, headquartered in Beijing, has modern radiopharmaceutical smart production bases in Jiangsu, Guangdong and Sichuan, and a branch office in the US, having formed business cooperation with more than a dozen multinational pharmaceutical companies. Relying on the world's leading R&D resources of radiopharmaceutical precision diogsis and therapy, Sinotau has taken the lead in laying out a number of targeted therapeutic and precision diagnostic radiopharmaceuticals in the fields of oncology, neurodegenerative diseases and cardiovascular disease, which are in or have completed clinical trials and will be launched in the coming years. Since April 2020, Sinotau has successfully closed nearly 1 billion RMB and has been favored by major investment institutions, with plans to land on major capital marekts in the near future.